- 69.41 KB
- 2022-06-16 12:02:16 发布
- 1、本文档共5页,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,可选择认领,认领后既往收益都归您。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细先通过免费阅读内容等途径辨别内容交易风险。如存在严重挂羊头卖狗肉之情形,可联系本站下载客服投诉处理。
- 文档侵权举报电话:19940600175。
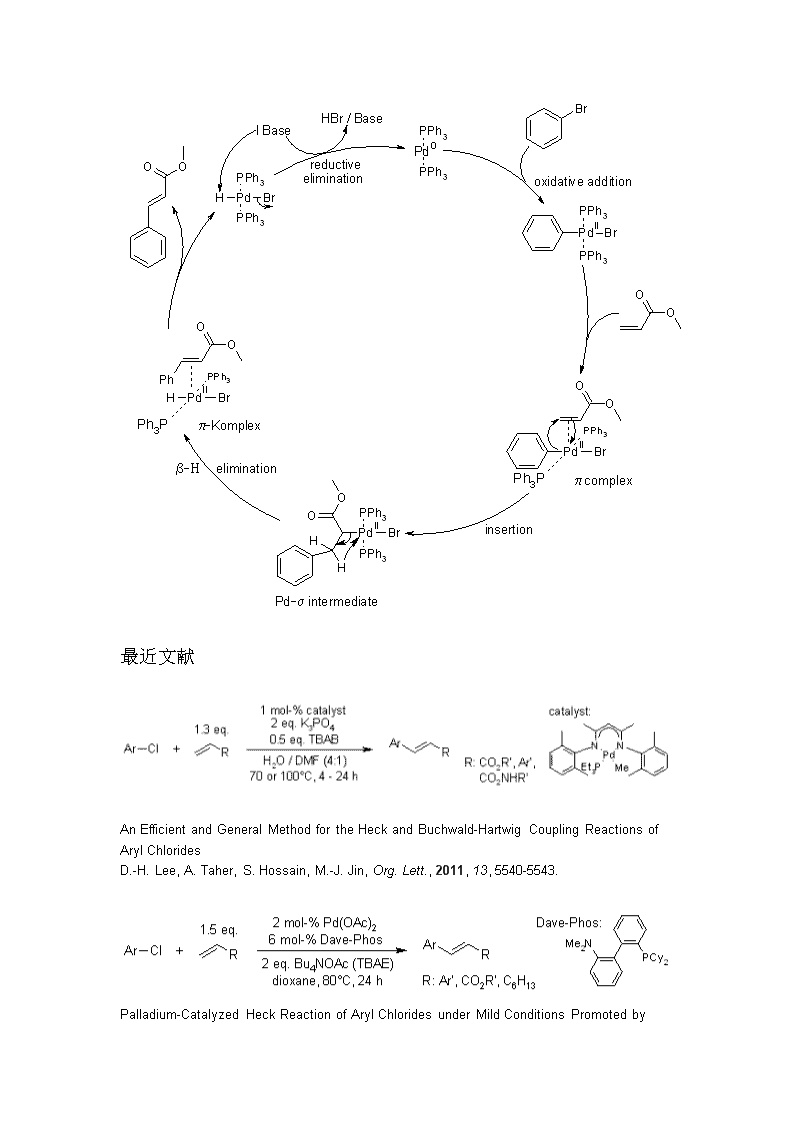
2010年诺贝尔化学奖获得者及其主要贡献美国化学家RichardF.Heck日本科学家根岸荣一和铃木章贡献①HeckReactionThepalladium-catalyzedC-Ccouplingbetweenarylhalidesorvinylhalidesandactivatedalkenesinthepresenceofabaseisreferredasthe"HeckReaction".RecentdevelopmentsinthecatalystsandreactionconditionshaveresultedinamuchbroaderrangeofdonorsandacceptorsbeingamenabletotheHeckReaction.OneofthebenefitsoftheHeckReactionisitsoutstandingtransselectivity.机理
最近文献AnEfficientandGeneralMethodfortheHeckandBuchwald-HartwigCouplingReactionsofArylChloridesD.-H.Lee,A.Taher,S.Hossain,M.-J.Jin,Org.Lett.,2011,13,5540-5543.Palladium-CatalyzedHeckReactionofArylChloridesunderMildConditionsPromotedby
OrganicIonicBasesH.-J.Xu,Y.-Q.Zhao,X.-F.Zhou,J.Org.Chem.,2011,76,8036-8041.EfficientAqueous-PhaseHeckReactionCatalyzedbyaRobustHydrophilicPyridine-BridgedBisbenzimidazolylidene-PalladiumPincerComplexZ.Wang,X.Feng,W.Fang,T.Tu,Synlett,2011,951-954.Anefficientandsimpleprotocolforphosphine-freeHeckreactionsinwaterinthepresenceofaPd(L-proline)2complexasthecatalystundercontrolledmicrowaveirradiationconditionsisversatileandprovidesexcellentyieldsofproductsinshortreactiontimes.Thereactionsystemminimizescosts,operationalhazardsandenvironmentalpollution.B.K.Allam,K.N.Singh,Synthesis,2011,1125-1131.①SuzukiCouplingTheschemeaboveshowsthefirstpublishedSuzukiCoupling,whichisthepalladium-catalysedcrosscouplingbetweenorganoboronicacidandhalides.Recentcatalystandmethodsdevelopmentshavebroadenedthepossibleapplicationsenormously,sothatthescopeofthereactionpartnersisnotrestrictedtoaryls,butincludesalkyls,alkenylsandalkynyls.Potassiumtrifluoroboratesandorganoboranesorboronateestersmaybeusedinplaceofboronicacids.Somepseudohalides(forexampletriflates)mayalsobeusedascouplingpartners.机理
OnedifferencebetweentheSuzukimechanismandthatoftheStilleCouplingisthattheboronicacidmustbeactivated,forexamplewithbase.Thisactivationoftheboronatomenhancesthepolarisationoftheorganicligand,andfacilitatestransmetallation.Ifstartingmaterialsaresubstitutedwithbaselabilegroups(forexampleesters),powderedKFeffectsthisactivationwhileleavingbaselabilegroupsunaffected.Inpartduetothestability,easeofpreparationandlowtoxicityoftheboronicacidcompounds,thereiscurrentlywidespreadinterestinapplicationsoftheSuzukiCoupling,withnewdevelopmentsandrefinementsbeingreportedconstantly.最近文献StereoconvergentAmine-DirectedAlkyl-AlkylSuzukiReactionsofUnactivatedSecondaryAlkylChloridesZ.Lu,A.Wilsily,G.C.Fu,J.Am.Chem.Soc.,2011,133,8154-8157
RecyclableCatalystsforSuzuki-MiyauraCross-CouplingReactionsatAmbientTemperatureBasedonaSimpleMerrifieldResinSupportedPhenanthroline-Palladium(II)ComplexJ.Yang,P.Li,L.Wang,Synthesis,2011,1295-1301AnEfficientandRecyclableMagnetic-Nanoparticle-SupportedPalladiumCatalystfortheSuzukiCouplingReactionsofOrganoboronicAcidswithAlkynylBromidesX.Zhang,P.Li,Y.Ji,L.Zhang,L.Wang,Synthesis,2011,2975-2983.EfficientPalladium-CatalyzedCross-CouplingReactionofAlkynylHalideswithOrganoboronicAcidsunderAerobicConditionsJ.-S.Tang,M.Tian,W.-B.Sheng,C.-C.Guo,Synthesis,2012,541-546.Ni(COD)2/PCy3CatalyzedCross-CouplingofArylandHeteroarylNeopentylglycolboronateswithArylandHeteroarylMesylatesandSulfamatesinTHFatRoomTemperatureP.Leowanawat,N.Zhang,A.-M.Remerita,B.M.Rosen,V.Percec,J.Org.Chem.,2011,76,9946-9955③NegishiCouplingTheNegishiCoupling,publishedin1977,wasthefirstreactionthatallowedthepreparationofunsymmetricalbiarylsingoodyields.Theversatilenickel-orpalladium-catalyzedcouplingoforganozinccompoundswithvarioushalides(aryl,vinyl,benzyl,orallyl)hasbroadscope,andisnotrestrictedtotheformationofbiaryls.
机理最近文献HighlySelectiveReactionsofUnbiasedAlkenylHalidesandAlkylzincHalides:Negishi-PlusCouplingsA.Krasovskiy,B.H.Lipshutz,Org.Lett.,2011,13,3822-3825.LigandEffectsonNegishiCouplingsofAlkenylHalidesA.Krasovskiy,B.H.Lipshutz,Org.Lett.,2011,13,3818-3821.
AnonionicamphiphileenablesasimpleapproachtoPd-catalyzedstereoselectivesp3-sp2cross-couplingsbetweenalkylandalkenylbromidesinthepresenceofzincpowderinwatertogivecoupledproductsingoodyieldswithoutpriorformationoftheorganozincreagents.Thereactionisconductedatroomtemperatureandtoleratesvariousfunctionalgroups.A.Krasovskiy,C.Duplais,B.H.Lipshutz,Org.Lett.,2010,12,4742-4744.Iron-CatalyzedNegishiCouplingTowardanEffectiveOlefinSynthesisT.Niu,W.Zhang,D.Huang,C.Xu,H.Wang,Y.Hu,Org.Lett.,2009,11,4474-4477.HighlyRegio-andStereoselectiveSynthesisof(Z)-TrisubstitutedAlkenesviaPropyneBromoborationandTandemPd-CatalyzedCross-CouplingC.Wang,T.Tobrman,Z.Xu,E.-i.Negishi,Org.Lett.,2009,11,4092-4095.HighlyRegioselectiveSynthesisofTrisubstitutedAllenesviaLithiationof1-Aryl-3-alkylpropadiene,SubsequentTransmetalation,andPd-CatalyzedNegishiCouplingReactionJ.Zhao,Y.Liu,S.Ma,Org.Lett.,2008,10,1521-1523.
您可能关注的文档
- Kraussmaffei Berstorff克劳斯玛菲.贝尔斯托夫公司及产品介绍
- 诺贝尔科学奖获得者寄语中国
- iPEARL爱贝尔
- (秋)2019一年级语文下册 课文4 第16课《小诺贝尔》教案 语文S版
- 2019一年级语文下册 课文4 第16课《小诺贝尔》教案 语文S版
- 达朗贝尔原理动静法1
- 呼伦贝尔CDMA网络_RSSI底噪异常问题排查处理报告0805
- 青草在歌唱(诺贝尔获奖作品)
- 化学人教版九年级上册《水的净化——和贝尔一起学净水》
- 5 马克思主义经济学与诺贝尔经济学奖
- 陆贝尔 莫氏庄园介绍
- 上海贝尔LGW VLAN模式设置方法
- 【 标 题】诺贝尔经济学奖二十五年概述
- 1901-2015诺贝尔化学奖得主(详细介绍)
- 1901-2012年历届诺贝尔化学奖得主与贡献
- 以苏教版小学语文《诺贝尔》备课为例
- 比较福禄贝尔与蒙台梭利资料
- 福禄贝尔学前教育思想资料